On November 27, a U.S. District Court for the Central District of Illinois allowed a class action lawsuit against Boston Scientific Corporation to continue. Judge Richard Mills of the District Court denied Boston’s motion to dismiss. The court found plausible claims with regards to the defective design and inadequate warnings on Boston’s inferior vena cava (IVC) filters.
This denial comes after Boston Scientific recalled about 18,000 IVC filters in 2005. The recall was initiated due to the filters alleged tendency to detach and cause embolisms.
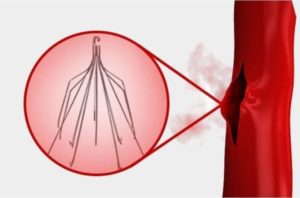
This lawsuit adds to a plethora of court decisions with regards to these controversial medical devices. IVCs are implemented just below the kidneys to capture blood clots, preventing them from going to the heart or longs.
IVCs were not approved by the U.S. Food and Drug Administration. The FDA received nearly 100 adverse effect reports related to the side effects of IVCs. From 2005 to 2015, the FDA issued multiple letters warning about the dangers of these devices and telling manufacturers that they were failing to warn patients of the risks associated with them.
Similar class action lawsuits against other IVC manufacturers are ongoing. These suits accuse companies of practicing negligence, concealment, and misrepresentation of data with regards to the negative effects the device could have on patients.


Laborde Earles injury was great for me they took care of me very fast and professional. If for any reason I need legal help they will be who I use.
ClientThe attorneys at Laborde Earles Injury Lawyers are on your side and ready to provide advocacy to anyone who has been injured due to the negligence of others. If you or a loved one has been victimized by a negligent manufacturing company, our dedicated attorneys are ready to fight for the just compensation your family deserves.