IVC Filter Safety Concerns
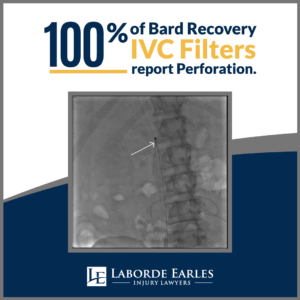
In 2010, the FDA released a safety communication due to reports of serious complications stemming from IVC filters. For some patients, the filter perforated the vein walls. In other cases, the device broke within the vein, causing components to flow to other areas of the body. Although the device is designed to reduce the risk of pulmonary embolism due to blood clots, broken pieces of the filter could themselves cause embolization. There is also increasing evidence that the device increases the risk of deep vein thrombosis.
These medical issues are more likely when the temporary version of the filter is not removed in a timely manner. Additionally, veins within the body do not have nerve endings, meaning that issues with the IVC filter may be asymptomatic.


Laborde Earles injury was great for me they took care of me very fast and professional. If for any reason I need legal help they will be who I use.
ClientReach Out to Laborde Earles for a Consultation
If you or someone you love is experiencing issues with their IVC filter, consider reaching out to legal counsel as soon as possible. The knowledgeable product liability attorneys at Laborde Earles Injury Lawyers understand the complexities of defective medical device litigation. Our dedicated lawyers could help you fight for fair compensation for your medical expenses, pain and suffering, and more. To schedule a consultation and discuss the merits of your claim, reach out to our office today.





